How Does The Photoelectric Effect Work?
Key Takeaway
The photoelectric effect works when light photons hit the surface of a material, typically a metal. These photons transfer their energy to the electrons in the material. If the energy is sufficient, the electrons are ejected from the material. This ejection is due to the photons providing enough energy to overcome the electron’s binding energy within the atom.
The amount of energy required to eject an electron depends on the material’s atomic number. Higher atomic numbers generally need more energy. This principle is used in various technologies, such as solar panels and photoelectric sensors, where light is converted into electrical energy or used to detect changes in light. Understanding this effect is fundamental to quantum physics and many modern applications.
The Science Behind the Photoelectric Effect
The photoelectric effect is a fundamental phenomenon in quantum mechanics where light striking a material surface causes the emission of electrons. This process occurs because photons, the particles of light, transfer energy to the electrons within the material. When the energy provided by these photons exceeds the binding energy (or work function) of the electrons, they are ejected from the surface. This effect provides crucial evidence for the particle nature of light, as it demonstrates that light can transfer discrete energy packets (quanta) to electrons. Understanding this effect is key to grasping the principles of quantum mechanics and the dual nature of light.
Interaction of Light and Matter
The interaction between light and matter during the photoelectric effect involves a precise sequence of events that are foundational to understanding quantum mechanics. When photons from a light source strike the surface of a material, they transfer their energy to the electrons within the material. The amount of energy a photon possesses is directly related to its frequency, described by the equation E=hνE = hnuE=hν, where EEE is the energy, hhh is Planck’s constant, and νnuν is the frequency of the light.
If the energy of the incoming photons is greater than the work function ϕphiϕ of the material — the minimum energy needed to release an electron from the material’s surface — the electrons absorb this energy. This absorption process enables the electrons to overcome the forces that keep them bound to the material. Consequently, these electrons are emitted from the surface, a process that occurs almost instantaneously as the photons impart their energy.
This phenomenon underscores the critical dependence on the frequency of the incident light rather than its intensity. Even light with low intensity but high frequency can cause electron emission, whereas high-intensity light with insufficient frequency cannot. This direct relationship between light frequency and electron emission highlights the quantum nature of light, demonstrating that light behaves not just as a wave but also as a particle with discrete packets of energy.
Role of Electrons in the Photoelectric Effect
Electrons are central to the photoelectric effect, acting as the particles that absorb photon energy and are subsequently ejected from the material. The photoelectric effect is a clear illustration of how electrons respond to different frequencies of light. When light with a frequency higher than the material’s threshold frequency hits the surface, the photons impart enough energy to the electrons to liberate them from their atomic bonds.
This ejection process is highly dependent on the frequency of the incident light. Higher frequency light, such as ultraviolet radiation, has more energetic photons that can free electrons even if the light’s intensity is low. Conversely, lower frequency light, such as visible or infrared light, may not have photons with sufficient energy to cause electron emission, regardless of the light’s intensity. This phenomenon demonstrates that the energy of the photons must meet or exceed the work function of the material to release electrons.
The role of electrons in the photoelectric effect also emphasizes the concept of energy quantization. It shows that electron emission does not occur continuously but in discrete energy packets, aligning with the principles of quantum mechanics. Understanding this interaction helps explain various technologies and scientific principles, including photovoltaic cells, photoelectron spectroscopy, and the fundamental behavior of electrons in different materials under varying light conditions.
Mathematical Representation
The mathematical representation of the photoelectric effect is captured by Einstein’s photoelectric equation:
Ek=hν−ϕE_k = hnu – phiEk=hν−ϕ
In this equation, EkE_kEk represents the kinetic energy of the emitted electrons, hhh is Planck’s constant (6.626×10−346.626 times 10^{-34}6.626×10−34 Js), νnuν is the frequency of the incident light, and ϕphiϕ is the work function of the material. The work function ϕphiϕ is the minimum energy required to eject an electron from the material’s surface. This equation illustrates that the kinetic energy of the emitted electrons is directly proportional to the frequency of the incident light, minus the material’s work function.
The significance of this equation lies in its explanation of the threshold frequency; only photons with an energy exceeding the work function can cause electron emission. If the photon’s energy is less than the work function, no electrons are emitted regardless of the light’s intensity. This relationship provided strong evidence for the particle nature of light, a fundamental concept in quantum theory. It demonstrated that light energy is quantized and can be considered as discrete packets called photons, each carrying energy proportional to its frequency. This understanding was pivotal in advancing quantum mechanics and revolutionized our comprehension of electromagnetic radiation.
Real-World Examples and Experiments
Numerous real-world examples and experiments demonstrate the practical significance of the photoelectric effect. One of the classic experiments is the use of ultraviolet light on a metal surface, where electrons are observed to be ejected, confirming that only light of sufficient frequency can cause electron emission. This experiment effectively demonstrated that the photoelectric effect is dependent on the light’s frequency rather than its intensity, aligning with Einstein’s theoretical predictions.
Modern applications of the photoelectric effect are widespread. In photovoltaic cells, which are the core components of solar panels, the effect is harnessed to convert sunlight into electricity. When sunlight strikes the photovoltaic material, electrons are emitted and captured, creating an electric current. This process of light-induced electron emission is critical for generating renewable energy from solar power.
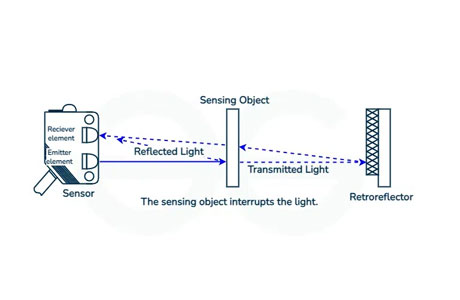
Another prominent example is in photoelectric sensors used in industrial automation and security systems. These sensors emit a light beam, and when an object interrupts this beam, the sensor detects the change and triggers an appropriate response, such as stopping a machine or sounding an alarm. These sensors rely on the photoelectric effect to detect objects and movements accurately and reliably.
These applications underscore the importance of the photoelectric effect in converting light energy into electrical signals, which is crucial for various technological advancements. From generating clean energy to enhancing automation and security systems, the photoelectric effect plays a vital role in modern technology and industry.
Conclusion
In conclusion, the photoelectric effect is a cornerstone of quantum mechanics that demonstrates the interaction of light and matter. By understanding the science behind the effect, including the role of photons and electrons, and its mathematical representation, we can appreciate its significance in modern physics. Real-world applications and experiments highlight its practical utility and ongoing relevance. The photoelectric effect not only deepens our understanding of light’s quantum nature but also drives technological advancements in energy conversion, sensing, and imaging. As we continue to explore and harness this phenomenon, its impact on science and technology will undoubtedly expand, offering new insights and innovations.
